The EU Medical Device Regulation 2017/745 (MDR) succeeds the EU Medical Device Directive 93/42/EEC (MDD), and medical device manufacturers of existing CE-marked devices under the MDD have a deadline of no later than 2028 (depending on the classification) to transition to the new MDR. In this step-by-step guide, we outline the technical documentation you need to prepare to facilitate a seamless transition.
- Checking the medical device classification
- Preparing a technical file
- Implementing a robust Quality Management System
What is the MDD to MDR transition period, and to whom does it apply?
The Medical Device Regulation came into effect on May 26, 2021, for new medical devices. But what about legacy medical devices?
There is an extended transition period for existing devices to achieve compliance:
- Class III custom-made implantable devices – deadline May 26, 2026
- Class III and class IIb implantable devices – deadline December 31, 2027
- Class IIb, IIa, and class I sterile/measuring devices – deadline December 31, 2028
This legislation applies to all medical device manufacturers operating within the EU, regardless of their country of origin. Medical companies operating in the United Kingdom are now obliged to receive UKCA certification instead of the CE marking, except for medical products sold in Northern Ireland.
Learn more about the EU MDR deadlines here: What are the new deadlines for medical device manufacturers to transition from the MDD to the MDR?
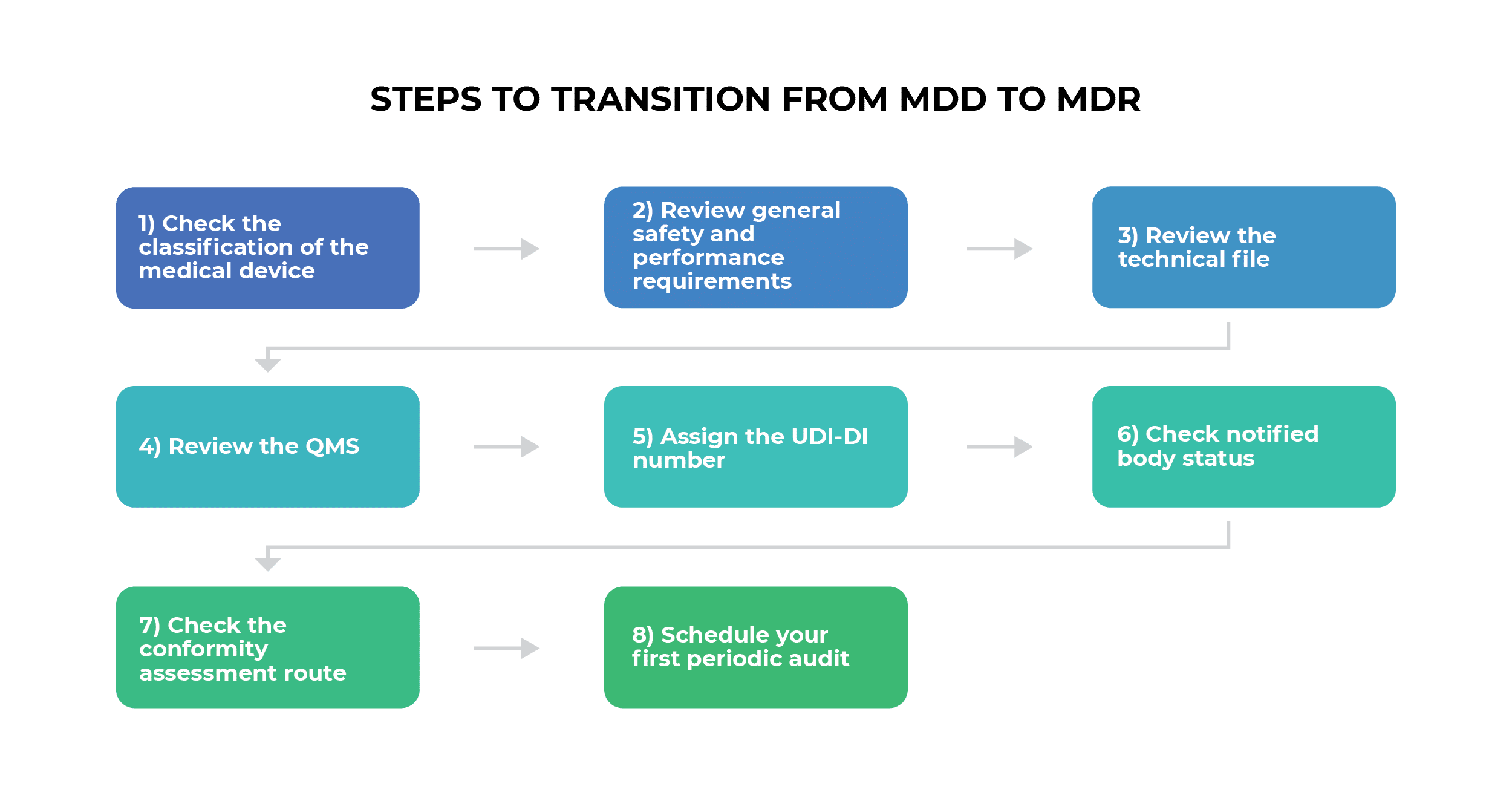
Eight-step transition process from the MDD to the MDR
Here are the eight steps that medical device manufacturers need to take when transitioning from the MDD to the MDR.
1) Check the classification of the medical device
The first step for medical device manufacturers is to check if their device is defined as a medical device in Article 2 under the MDR classification, because the scope has changed significantly compared to the MDD.
For instance, the terms “prediction” and “prognosis” of disease have been added to the definition of a medical device. So, any device that serves to predict a disease is now considered a medical device. The same is now the case for devices that are used to clean, disinfect, or sterilize medical devices.
Other additions are:
- Products that need to be reprocessed (Article 17)
- Products without an intended medical use (Annex XVI)
Active implantable medical devices were previously covered by a separate directive (90/385/EEC) but are now covered by the MDR.
Learn more about the changes here: EU MDR vs. MDD – What has changed?
2) Review general safety and performance requirements
Once the medical device has been classified, the manufacturer needs to review the device’s safety and performance requirements in accordance with Annex I of the MDR, which outlines the general safety and performance requirements (GSPR).
- Chapter 1 covers the requirements of technical files and QMS documentation.
- Chapter 2 covers the requirements regarding design and manufacture.
- Chapter 3 covers the requirements regarding the information supplied with the device.
Not all requirements in these chapters are applicable for all types of medical devices. For example, if a medical device does not require other devices, equipment, or electricity, then point 14 (Construction of devices and interaction with their environment) won’t be applicable.
Compliance with GSPR is often achieved through the use of a checklist or table, with columns for applicability, justification, and the method or standard that refers to a particular requirement. If a requirement is not applicable, you must clearly describe the justification so that a third party can understand your reasoning.
Leverage ISO 13485 as the base for QMS requirements in MDR compliance: How can ISO 13485 help with MDR compliance?
3) Review the technical file
After reviewing the general safety and performance requirements, manufacturers need to review the technical file. Annex II describes the technical documentation required, and Annex III covers the technical documentation required for post-market surveillance.
The MDR does not explicitly state how technical documentation should be structured; it only states that it should be “presented in a clear, organised, readily searchable and unambiguous manner.”
Elements that must be covered in the technical documentation according to Annex II include:
- Device description and specifications, including variants and accessories
- Information to be supplied with the device, such as packaging labels and instructions
- Design and manufacturing information
- General safety and performance requirements
- Benefit-risk analysis and risk management
- Product verification and validation
For implantable devices and class III devices, the manufacturer must create a short report on the safety and clinical performance (Article 32). According to Article 10, technical documentation must now be archived as follows: class I and II devices (except implantable devices) after 10 years, and class III and implantable devices after 15 years.
See more about what technical documentation is required here: What are the EU MDR technical documentation structure and requirements?
4) Review the QMS
After reviewing the technical file, medical device manufacturers must ensure that they have ISO 13485:2016 implemented according to the MDR. The good news is that MDD-compliant manufacturers have already implemented a Quality Management System (QMS) under ISO 13485:2016.
MDD-compliant manufacturers — that is, those who have already implemented ISO 13485 — only need to add seven additional requirements to be compliant with the MDR:
- Strategy for regulatory compliance (Article 15)
- Identification of safety and performance requirements (Annex I)
- Clinical evaluation (Article 61 and Annex XIV)
- Verification of the UDI assignments (Article 27 and Article 29)
- Post-market surveillance (Article 83)
- Communication with economic operators (Article 11)
- Field safety corrective actions (FSCA) in the context of vigilance
Medical device manufacturers that have not yet implemented ISO 13485:2016 must prepare their Quality Management System according to the ISO 13485 standard, together with the additional MDR requirements stated above.
See here for more information on the importance of QMS requirements: What is ISO 13485?
5) Assign the UDI-DI number
Once the QMS has been reviewed, the manufacturer needs to obtain a unique device identification device identifier (UDI-DI) from an official designated entity, such as GS1, HIBCC, or ICCBBA.
After receiving the UDI-DI, the manufacturer needs to place it in the following documentation:
- Declaration of Conformity (to be completed in Step 7)
- Technical documentation
- Summary of safety and clinical performance
- Certificate of free sale (Article 60)
A UDI is essentially a barcode that allows devices to be tracked in the EU and allows for better monitoring by the competent authorities. Devices must be registered in the EUDAMED database.
6) Check notified body status
After the registration of the device, it’s necessary for manufacturers to contact their notified body and check if they are designated for the MDR. Not all notified bodies that operate under the MDD will operate under the MDR.
The complete list of currently designated notified bodies can be found here on the European Commission website (wait for a couple of seconds for the page to load).
7) Check the conformity assessment route
A conformity assessment is required once the notified body’s status has been checked. This is a procedure that determines whether a medical device complies with the requirements of the MDR. It’s carried out to prove that general safety and performance requirements are fulfilled.
According to the MDR, there are three types of conformity assessment procedures:
- Conformity assessment based on a Quality Management System and the assessment of technical documentation (Annex IX)
- Conformity assessment based on type examination (Annex X), where a notified body certifies that a device complies with the regulation
- Conformity assessment based on product conformity verification (Annex XI)
All classes of medical devices must prepare a Declaration of Conformity in accordance with Annex IV. The only exception is for custom-made devices that must prepare a Declaration of Conformity according to Annex XIII. A medical device manufacturer can affix a CE marking on their device after a successful conformity assessment.
8) Schedule your first periodic audit
After the conformity assessment, the manufacturer’s first periodic audit must be scheduled, which must be completed while they still have a valid MDD certificate. According to Article 120, manufacturers must be prepared with the following:
- Post-market surveillance
- Market surveillance
- Vigilance
- Registration of economic operators
Learn more about what you should do at the certification audit: How to approach an auditor in a certification audit.
MDD to MDR transition
While the transition from MDD to MDR might seem daunting, it’s important to remember that this change is designed to enhance patient safety and product quality. Embracing the transition can lead to improved healthcare outcomes and greater trust from patients. With careful planning, thorough preparation, and a commitment to compliance, the transition can be managed successfully.
To transition to the EU MDR in the fastest and most efficient way, check out this ISO 13485 and EU MDR Integrated Documentation Toolkit.

 Kristina Zvonar Brkic
Kristina Zvonar Brkic