When you think about Quality Management System (QMS) documentation, do you picture piles of documents? Maybe bureaucratic red tape and unnecessary procedures? For some companies, this is the unfortunate reality, because they mistakenly believe that the more documents they create – the more compliant they will appear to be. Don’t let your company fall into this trap.
Of course, being the international standard for Quality Management Systems in the medical device industry, ISO 13485 does require certain documentation (see this article: List of Mandatory Documents required by ISO 13485:2016). But, that documentation serves a number of purposes – and not one of them involves making your company simply appear to be compliant.
The real purpose of QMS documentation is threefold:
- to provide a clear framework for the company’s operations
- to facilitate process consistency and better understanding of the Quality Management System
- to show evidence of the company’s achievement of its goals and objectives
So, when you set out to create your QMS documentation, your focus should be on efficiency, and on creating only those processes and documents that will benefit your organization.
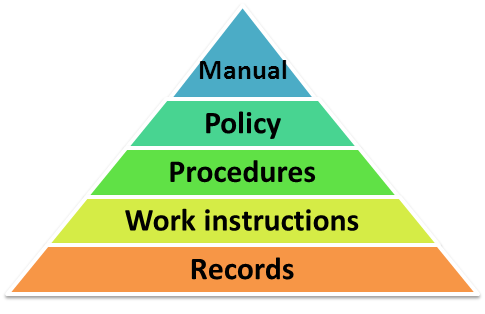
QMS documentation hierarchy
A typical QMS will contain a variety of documents, some of which include:
- Quality Policy
- Quality Manual
- Procedures and Work Instructions
- Quality Plans and Records
The hierarchy of this QMS documentation is depicted in the diagram below:
The ISO 13485 standard requires various types of information to be documented; but, not every piece of information has to have its own separate document. The standard allows an organization the flexibility to decide on the amount of documentation it needs, and the level of detail to include. For example, small companies may choose to document a simple overview of their procedures in the Quality Manual.
How to structure your QMS documentation
There is a separate standard, called “ISO 10013:2001 Guidelines for quality management system documentation,” which offers some direction as to the number and size of the documents your organization will likely need. It also provides guidance on the structure and contents of each document. The following is a summary of some of these ISO 10013 guidelines.
Quality Manual. Clause 4.2.2 of ISO 13485:2016 states that the Quality Manual should include those clauses that are applicable to the organization, with a reason given for any clauses omitted. The contents of the manual, as well as the overall structure, are going to be dependent on the characteristics of the organization itself, such as size, operational complexity, and staff competencies. While a small company can probably document their entire Quality Management System in a single manual, a large multi-national company may need several Quality Manuals, plus all the other required documents.
A typical Quality Manual will include:
- title and table of contents
- information about document version and approvals
- a description of the QMS
- the scope of the QMS
- any exclusions from ISO 13485, and the reasoning behind them
- the company’s business process model
- roles and responsibilities of personnel
- references to additional relevant documents and appendices
- the Quality Policy and objectives
You can learn more about how to write an effective Quality Manual in this article: ISO 13485: How to write a short quality manual.
Quality Policy. The Quality Policy is a statement of the company’s commitment to quality, to continual improvement, and to fulfilling its legal and regulatory obligations. Basically, it outlines your organization’s quality goals, which are defined by quantifying its quality objectives. Some companies choose to use the Quality Policy for promotional purposes; you’ll want to display it on the premises and on the company website, so keep it simple and concise.
Quality procedures. The quality procedures are the very backbone of an organization’s QMS. Their purpose is to establish processes that will ensure the company’s activities conform to ISO 13485 requirements. Quality procedures come in all shapes and sizes. They can be descriptive, such as in narrative form; they can be in a more structured format, like tables; they can be more illustrative, such as a flow chart; or they can be a combination of any or all of the above.
Quality procedures should include the following elements:
- the title – to identify the procedure
- its purpose – the reasons for the procedure
- its scope – to define what is included in the procedure, and what is not
- roles, responsibilities, and authorities of those involved in the procedure
- a list and definition of records that result from the activities described in the procedure
- identification of changes, date of review and approval, and version of the document, in accordance with the established practice for document control
- a description of the activities in the procedure (this is the main body of the procedure) – describing what should be done, how, when, where, and by whom. In some cases, the “why” should be clarified as well, plus the inputs and the outputs of the activities, including the necessary resources.
- appendices, if needed
Work instructions. You can include work instructions as a part of a procedure, or simply reference them in the procedure. Work instructions are typically structured in the same way as the procedures, and cover the same elements; but, the work instructions provide greater detail about the activities that need to be performed, with an emphasis on the sequence of steps to be taken, the tools and methods to be used, and the accuracy requirements. They can also include provisions for any risk related to the activity, how to mitigate it, and its potential impact on safety, performance, and compliance with regulatory requirements if not managed properly.
The use of competent personnel, and adequate staff training, will eliminate the need for highly detailed work instructions. You can learn more on this topic in the article Using Competence, Training and Awareness to Replace Documentation in your QMS.
Records. These are the low-level documents that provide evidence that a process is in place and performed according to the procedure or work instruction. For example, inspection records show that an inspection was performed, along with some specific findings.
Good QMS documentation is essential for an effective Quality Management System
Planning the size and scope of your QMS documentation based on your organizational needs is essential for a functional and efficient Quality Management System. Moreover, properly structured documentation will make your operations much easier, while sloppy documentation will bring you nothing but trouble.
Click here to download a free white paper Checklist of Mandatory Documentation Required by ISO 13485:2016.

 Waqas Imam
Waqas Imam