 Waqas Imam
Waqas Imam
September 28, 2017
Human factors are one of the most important considerations in the design of medical devices, and are reflected in an ISO 13485 Quality Management System as design and development inputs.
Human factors to consider include the requirements of device users, the environment where the device is being used, and the interface of the user with that device. Only the correct incorporation of all three considerations can ensure the safe use of the medical product. If these factors are not incorporated properly in the design phase, then the result may be reduced quality or defective product, decreasing its functionality. Human Factors Engineering in medical device design is also known as Usability Engineering.
The main purpose of the consideration of human factors is to reduce product application hazards and associated risks. A secondary focus is making sure that device design outputs accommodate all of the defined factors in the inputs, so that users can deploy the device safely and efficiently. Some of the benefits of human factors consideration in device design include easy application of devices, safer electrical connections, easy-to-understand displays, easy interpretation of device functionality, easy understanding of the patient’s medical state with the device, etc. Moreover, through incorporating these factors in design, companies can avoid product recalls, complaints, lawsuits, and other adverse events. The U.S. Food and Drug Administration (FDA), mandates use-related risks to be recognized, controlled, and validated for premarket submissions.
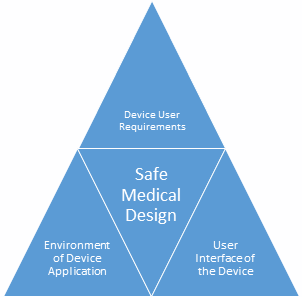
Many decades ago, considering humans factors during the design process was not a developed methodological process, but now human factors and design controls are inseparable from each other. The following are three considerations for medical device design:
2) Environment of device application: Medical devices are applied in an environment that may have numerous conditions that can affect usability. Medical devices might be utilized in clinical and non-clinical environments, community locations, or moving automobiles. Some environmental use situations are listed below:
3) Device user interface: A device user interface incorporates all areas of interaction between the user and the device, together with all features with which the operator works. A device user interface starts from installation of the device (e.g., installation, calibration), operation of the device, and maintenance on the device (e.g., replacing a battery, fixing parts). It involves:
Human factors are incorporated into the medical device design and development phase with the help of a risk management program. Considering the user requirements, environmental conditions, and the device user interface, all hazards related to product safety and functionality are identified (risk assessment is performed). The risks exceeding the acceptable limit are controlled with the help of risk mitigation plans. The residual risk is calculated and brought under the acceptable limit. This is how medical device safety is ensured.
Regulatory bodies are making it mandatory for medical device manufacturers to consider human factors in their device design, and also in their risk management program. The FDA requires a Human Factors Engineering or Usability Engineering report to be submitted in the premarket submission process for a medical device. With the help of the ISO 13485 design and development program and the ISO 14971 risk management program, medical device manufacturers can effectively incorporate human factors in the device design and can mitigate risk hazards concerning human factors.
Use this free Diagram of ISO 13485:2016 Implementation Process to integrate the medical device design process in the overall implementation.